Home > Products > Dryalpha special feature

Corrosion inhibitor
The corrosion inhibitor (inhibitor) means an inorganic or organic medicine decreasing metal corrosion remarkably by adding it in small quantities in corrosion environment. It is studied as a corrosion inhibitor conventionally, and, for documents, it is announced many as a product again, and the discovered material is commercially available.
The corrosion inhibitor has been developed for a purpose not only to prevent metal corrosion in a water solution, acid solution, an alkaline solution, organicity solution as corrosion environment, but also prevent atmospheric corrosion in the wide range.
The corrosion inhibitor was old, and the suppressant of the inorganic system was used, but I announced that Marangoni and Stephonelli helped that an organicity material from essential oil prevented corrosion of iron with the acid in about 1872, and the study of the organic suppressant was done.
It was known that the organic compound which contained nitrogen, arsenic, phosphorus, sulfur in the next 1,920 years was an effective corrosion inhibitor.
Because it is different each, about the restraint mechanism of the corrosion, as for the behavior of the suppressant, it is difficult by the kind of the corrosion inhibitor, corrosion environment namely a corrosion solvent, metal to elucidate the restraint mechanism of the suppressant generally.
Therefore, various kinds of opinions are submitted to restraint mechanism for the metal of the corrosion inhibitor.
It is distinguished in an adsorption theory, an overvoltage theory, a film theory when I classify roughly, and soaked in theory of the restraint mechanism that stood on the corrosion theory that is electrochemic as a thing forming these roots is done.
On the other hand, about the corrosion restraint mechanism that I cannot elucidate in this, there is a theory by the passivation by the chemical bond, and it may be said that both are the main constituents of the restraint mechanism. I may express restraint mechanism by each polarization action still more definitely whether a suppressant influences an anodal reaction electrochemically, or you influence a cathodal reaction.
| Generally electrochemic interpretation is accomplished, and the next reaction produces the metal corrosion in the cathorde district. |
In the anodal (anode) district. |
 | |
Mann adsorbs mercaptan including aliphatic amine including nitrogen, aromatic
amine, Heterocyclic Nitrogen compounds and sulfur, aldehyde including oxygen, the ketones in
acid solution with carbon chains compound having a polar group in the metal
surface in 1936, and, with a thing indicating the restraint action, these
suppressants ionize in oniumion which did an electric charge easily in
+ and adsorb it in the cathode district of the metal surface and form an
adsorption film and announce that I prevent metal corrosion from acidity
solution.
The corrosion restraint performance of the film which I adhere to the metal surface, and was produced raises overvoltage by preventing the electric discharge of the H ion, and, with the thing by the effect to prevent metal elution from the one anode, it is influenced by the polar group of the molecules of the suppressant, molecules sequence, molecules density and adsorption speed.
I generate a film in the anode district that I adhere to the metal cathode district in the suppressant and influence a cathode reaction, and there is a thing preventing metal corrosion. Aliphatic amine and aromatic amine prevent a cathode reaction as an example and raise hydrogen overpotential.
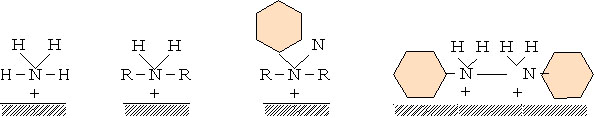
This adsorption film forms a monolayer.
The performance varies according to the molecular weight of these amines and the molecular structure, but generally shows the next order.
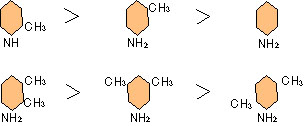
propylamine > ethylamine > methylamine > xylidine > toluidine > aniline
As for the second grade amine, the corrosion restraint performance is superior to the first grade amine, and the third grade amine is higher-performance.
N(CH3)3>H2NC3H7 N(C2H5)>HN(C3H7)2
The restraint performance of each compound of iso-chain and normal chain are inferior to the latter in the former generally.
With the aromatic series the performance as follows.
I explain a suppressant letting you insulate metal by the generation of the film which is minute in a metal surface from corrosion environment.
As for this, the organic sulfur compounds neutral or in the inorganic suppressant in the alkaline solution, e.g., chromate, corrosion restraint action of iron with the condensation phosphoric acid salt and acid solution generate the film of metal and the infusibleness compound and prevent metal corrosion.
The corrosion of iron in the water solution with chromate is regarded as a film by the generation of γ-Fe2O3 caused by the oxidation of the iron surface.
This is expressed in the next equation.
2Fe + 2Na2CrO4 + 2H2O → Fe2O3 + Cr2O3 + 4NaOH
The condensation phosphoric acid salt forms metal and a complex in the cathode district of the metal surface and produces an insoluble protection film.
Mann, Lawer & Multin was added inhibitor of amine in the sulfuric acid
solution, after the iron specimen was 16 hours immersed in it, was placed
in the added non sulfuric acid solution of inhibitor, and by measuring
the change over time in performance, are shown in the table below the results
were discussed amine-based film generation.
| Immersion liquid depressive | Control rate(%) | ||
| Dimethylamine | Dibutylamine | Triethylamine | |
| Acid solution without four hours after the suppression agent Acid solution without eight hours after the suppression agent Acid solution without sixteen hours after the suppression agent |
99.26 99.21 99.19 76.27 |
98.4 96.9 92.7 74.8 |
99.31 99.74 79.24 71.32 |
Amine-based inhibitor in hydrochloric acid solution I react with ferric chloride, in methyl amine[CH3NH3][FeCl4] nH2O,
in trimethylamine it can generate a protective coating of insoluble compounds [(CH3) 3NH] [FeCl4] nH2O have been published.
Lenny Hugel when you use the mercaptan as an inhibitor of iron in an acidic solution, has announced that it resulted in a film of iron sulfide.
Is analyzed by such adsorption theory, overvoltage theory, and film theory for corrosion inhibition mechanism of more metal.
Corrosion atmospheric metals conventional paint, plating, has been prevented from advancing corrosion coating for corrosion resistant coating a metal surface.
Systematic study of the corrosion in the atmosphere have been made by Vernon.Impurities corrosion in the air and moisture in the air I have shown that progression.
Vernon also dust in the air was tested whether affect corrosion.
Results of examining the progression of corrosion in the top of the atmosphere uncoated and the coated iron in Moss cloth, corrosion uncoated iron piece is to obtain a significantly greater results.
Also for CO2, SO2, H2S, NO2 content of atmospheric impurities, such as many in the industrial area, is further accelerated corrosion in proportion to the increase of its concentration.
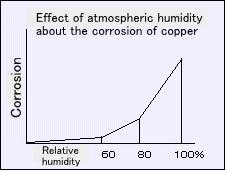
In the air close to the shore because it contains NaCl mist, corrosion is increased.
Corrosion inhibitors for these corrosio;n in the atmosphere is approximately divided into the following two.
1) non-volatile inhibitor
2) volatile inhibitor
non-volatile inhibitor also be distinguished in the next two.
a)contact inhibitor : Those that act in contact with the surface of the metal.
b)spreading inhibitor : Inhibitors rapidly diffuse into the moisture of the metal surface.
volatile inhibitor is intended to prevent corrosion of metal in gas phase or vapor, also referred to as vapour phase inhibitor with high inhibitor vapor pressure.
It is impregnated in the wrapping paper, and evaporated to a space wrapping paper and the contents thoroughly space is saturated with steam, to prevent corrosion of the underlying metal.
volatile inhibitor in nitrite organic compound in the first Shell's UK patent, vaper presser of this inhibitor was in the range of 0.0002 ~ 0.001mm / Hg at 21 ℃.
As typical, trimethylsulphonium is raised.
The organic esters and organic esters thio nitrite nitrite also has this kind of control performance, especially dialkylamine nitrite and morpholine nitrite showed good performance.
The Soviet Union in the diisopropylammonium nitrite, and published for dicyclohexyl-ammonium nitrite, former iron, chromium, Monel, and tin is effective in, copper and copper alloy, silver, aluminum, antimony, lead, opposite to the bad suppression in zinc I showed the performance.
dicyclohexylammonium nitrit is melted at 154.4 ℃, odor and is used, for example, may be saturated rust grease rust paper space at 566m at room temperature without 1g.
1.7% diisopropylammonium nitrite is the + 0.4% etanolamine salicylate or 0.1% sodium mercaptobenzthiozale exhibit excellent performance.
According to the data of Wachter & Skeis mixture of alkylamine salt
of alkylamine nitrite and carboxylic acid is a volatile corrosion inhibitor.
Cyclohexylamine carbonate in the UK patent iron, zinc, and the chrome plating has the effect of corrosion inhibiting, copper, magnesium, no corrosion inhibiting effect on cadmium.
Balezin, & Barannik the results of testing by impregnating the monoetanolamine
carbonate in wrapping paper and grease corrosion prevention of metals in
the atmosphere, are excellent in corrosion for iron, copper, nickel, and
the alloys rather corrosion me occurred.
dicyclohexylamine carbonate showed iron, iron alloy, copper and performance of corrosion also blocked by the copper alloy.
Quite many different things with these in the case of anti-corrosion Technology Handbook (Okuda Author) the electrical contact material we excerpt from as reference for the corrosion inhibitor, also because it contains such as poison / carcinogens, the experiment Please be careful as if you are being.