Home > Report > Report/Action of Corrosion Gas

Gas Corrosion Test And the Accelerated Rate
Kainetex:Makoto Sano
Report
- Gas Corrosion Test and the Accelerated Rate
- Introduction of temperature-and-humidity-control record and gas concentration control
- Action of Corrosion Gas
Various environmental tests have been conducted in order to evaluate the
long term reliability for the contact mechanism parts; such as switches,
connectors, etc.
In these tests, the gas corrosion test is supposed to be the most adequate
one. However, since we have numerous gas corrosion specification, we get
quite a few questions on the number of the accelerated coefficient in these
specifications, in comparison with that of the actual environment.
We will be able to respond up to certain point from what we have experienced
in the past. Unfortunately, we won't be able to provide any data for that
due to the need for further research.
Below is a question received from one of our customers:
"We found an intense corrosion in a recent test that we didn't find
in the previous test. It must be something wrong with the test equipment."
As a response to this question, we can say that there is a case that the
corrosion level slightly differs by how the parts are set in the test chamber.
Furthermore, we can also give you some greater factor as follows.
The corrosion of plating samples among same lot appears differently.
Still less the different lots with different production date by several
months can not be compared. Therefore, the current test should be taken
as a comparison test and you should not have confidence in the previous
test. It is important to think the gas corrosion test as a part of quality
control.
Natural environment contains small amount of various types of gas. In fact,
the corrosion gas which has high concentration exists in some areas. Also,
if a primary factor causing the corrosion gas exists in the sealed container,
the corrosion gas can be occurred as if it is not sealed up.
Although we have stated at the beginning that the gas corrosion test is
adequate to evaluate the reliability of the contact mechanism parts, the
single gas and mixed gas will have a significant difference in the test
result. In general, the hydrogen sulfide in Silver and Copper have a characteristics
of strong corrosiveness and as for the gold plating, sulfur dioxode and
nitrogen dioxide, which will turn to acidity with moisture, are recommended
for use. However, despite the fact that the amount of the concentration
is low, nitrogen dioxide, sulfur dioxide, hydrogen sulfide, chlorine, ammonia,
holmaldehyde, etc. are existing in the actual environment, and also they
are generated from the peripheral materials. From the above, if the low
concentration three kinds of mixed gas test which is specified in the Batel
Standard is conducted with exposure to the single gas fully, the usage
of three kinds of mixed gas will have an excellent reproducibility in the
natural environment. But, there are some parts that are used in a special
condition that the test temperature is high and the concentration is high
and so forth. At the same time, as the contact mechanism parts are originally
functional parts, some of them are easily affected by gas due to characteristics
other than then the environment usage. Therefore, you can see that it isn't
easy to discuss the accelerated coefficient for the Contact reliability,
including the cases being studied and implemented by people in the field
for your information. In general, in order to take the basic data, the
actual parts should be set at some places indoor and outdoor and left for
at least ten years. Meanwhile, numerous data has been collected many years,
which has been used the base for the data. However, the data has not been
completely established yet. (Although Bell Laboratory should have accumulated
numerous data in the past, there was a small amount of feedback.)
This attributes to the characteristics of the contact mechanism parts,
which we feel can't be avoided. The reason is that the contact mechanism
itself is very sensitive. For example, when the relation between the contact
resistance and gas corrosion test of copper plate is measured, the rate
goes up considerably close to the rate at the peak in the gas corrosion
test run for the several hours. Also, the result of the chlorine test on
a pure silver sample for the same measurement showed the same increased
rate as the above gas corrosion test. As for the gold plating sample, the
corrosion differs partly, and the evaluation of the contact mechanism will
be considerably complicated. From these factors, it is nearly impossible
to make a sample that doesn't vary widely and could be used as the standard.
In fact, quite a few of sufide of Copper and Silver can be seen on the
pure gold plate(99.9999%). So, from the standpoint of the contact resistance,
it should be taken into consideration in using it as a standard sample.
Further, ther are numerous "know-how" techniques for measuring
the contact resistance evaluation, lying such sensitive coating, without
destroying the coating. It will be more complicated for the samples which
can determine the quantity to a certain number when they are turned into
the actual parts to be used. Because, it is thought that the influence
of gas corrosion will vary considerably due to the mechanism, material,
variation, etc. Besides, it is also quite difficult to grasp the change
of contact resistance using the actual parts.
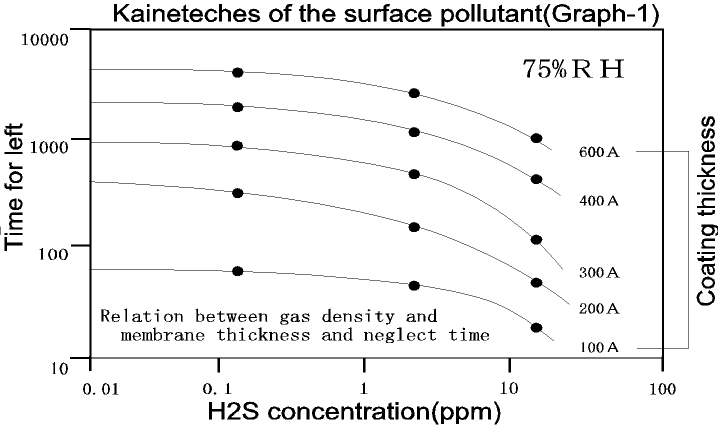
The first graph indicates the relation between the gaseous concentration(H2S), the coationg thickness and the time for left. As you will see from
the graph, it is wrong to think that the thickness of coating will increase
in proportion to the concentration. A difference is seen when the coating
is slowly produced is low concentration and when the coating is quickly
produced in high concentration using the contact resistance. Furthermore,
a comparatively large difference is seen between the single gas and the
mixed gas.
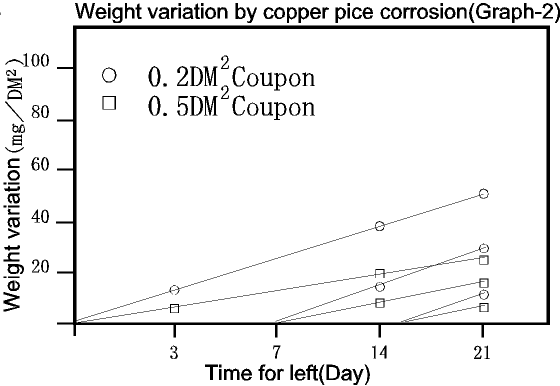
Following Graph-2 indicates the weight variation of the corrosion of the
weight of the specified metal piece after pre-treatment measured using
the high sensitivity scale. Although it differs by the square measure of
the test sample, it seems possible to use as a monitor of the corrosion
test if the condition such as the pre-treatment and the materials are thoroughly
reviewed. However, it is possible that there might be difficulty in finding
the correlation under the test condition that generates salts.
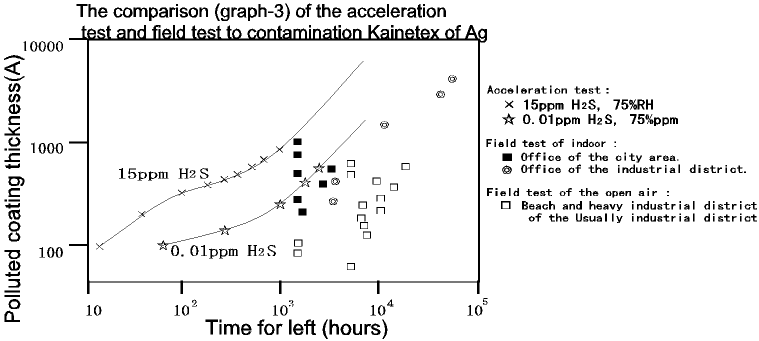
Following Graph-3 indicates the rustle of correlation between the accelerated
test and the field test evaluating by the thickness of coating. The field
data to the following possible courses:
- the humidity differs by location
- the concentration of hydrogen sulfide differs in the atmosphere
- the variation of temperature
- mixture of the dust and other gases
- the purification level of the trial material surface and the initial surface
- the purity of the trial material (mixture of Cu, Fe)
- whether or not the test site gets sunshine
Based on this data, if the condition of the hydrogen sulfide concentration
is 0.01 ppm and the humidity is 75% RH as the field data, the accelerated
rate of the accelerating test for 15 ppm hydrogen sulfide is as follows.
If the thickness of coating is specified as 300A, it will take 100 hours
for 15 ppm concentration of the hydrogen sulfide, and 1000 hours for 0.01
ppm concentration. Therefore, the ratio is simply 10, and for the actual
parts, the coating has a strong break-down so that the test can not be
difficult.
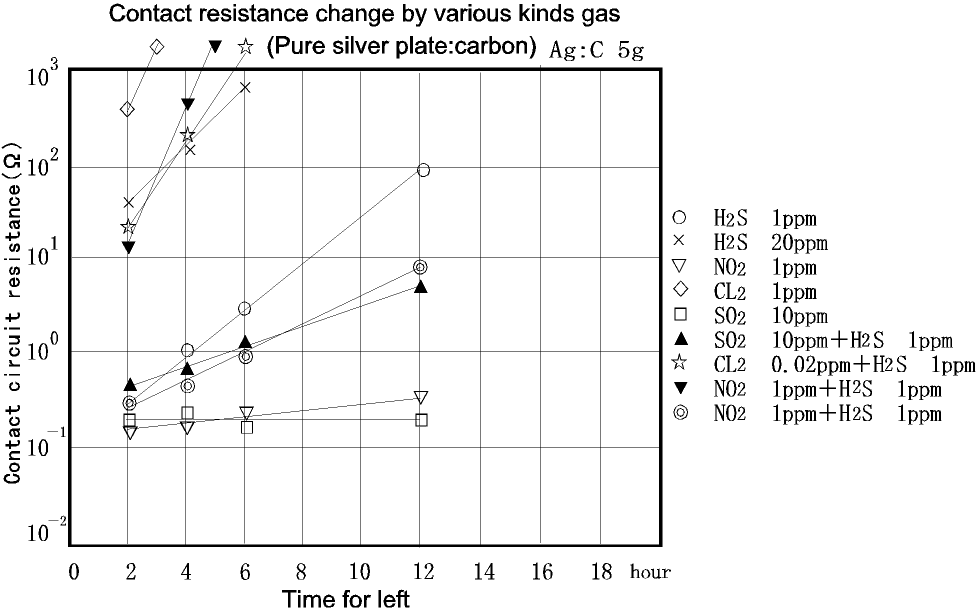
Data on the contact resistance using mixed gases without distracting the coating is shown on the last Graph-4.
References: Basic of the contact technology and contact reliability , improvement
and measures
Mr. Terutaka Tamai, Hyougo University of Education
Operation
requirements for accelerated gas testing using low concentrations of flowing
gas S A Harris ITT Cannon UK, Basingstoke