Home > Report > Corrosion Gas Action

Action of Corrosive gases such as Hydrogen sulfide and Sulfur dioxide.
Report
- Gas Corrosion Test and the Accelerated Rate
- Introduction of temperature-and-humidity-control record and gas concentration control
- Corrosion Gas Action
Sulfur dioxide(SO2) itself does not have strong corrosiveness, but when
humidity goes up,
H2O acts to be H2SO3(acid).
◆Reaction of a formation substance appears on the surface of Gold(Au).
H2O acts to be H2SO3(acid).
◆Reaction of a formation substance appears on the surface of Gold(Au).
- SO2+H2O ----->H2SO3(sulfurous acid)
- SO2+NO2 ----->NO+SO3
- SO3+H2O ----->H2SO4(sulfuric acid)
- H2SO4+NH3--->(NH4)2SO4(sulfuric ammonium)
◆Example of metal salt which can be formed on copper, nickel base gold-plated
a surface: NiSO4, CuSO4, NiNO3, Cu(NO3)2, and other metal salt.
a surface: NiSO4, CuSO4, NiNO3, Cu(NO3)2, and other metal salt.
Hydrogen (H2S) is gas that corrodes silver(Ag) and copper(Cu) severely.
In the case of H2S and Ag, the reaction formula is as follows:
In the case of H2S and Ag, the reaction formula is as follows:
- Ag -----> Ag + e
- 2H2S + O2----->2S + H2O
- S + 2e----->S -
- 2Ag + S- ----->Ag2S
- ※4Ag + 2H2S + O2----->2Ag2S + 2H2O
- ※2Cu + 2H2S +O3----->2CuS + 2H3O
The contamination mechanism of metal greatly affected by water so that
after water level gets to 70% RH,
it will be wet corrosion.
The humidity in atmosphere changes from dry corrosion to wet corrosion, which means that drops of water will be
formed as the result of large water absorption.
In addition, the vapor grains become greater in the atmosphere.
Because of this, the contamination gas tends to dissolved in this kind of water, which will be mist. (An electrolytic
solution adhered on metal surface.)
Then, metal becomes ion that dissolves into solution, which forms a contaminated substance by reacting to soluble ion in the solution.
it will be wet corrosion.
The humidity in atmosphere changes from dry corrosion to wet corrosion, which means that drops of water will be
formed as the result of large water absorption.
In addition, the vapor grains become greater in the atmosphere.
Because of this, the contamination gas tends to dissolved in this kind of water, which will be mist. (An electrolytic
solution adhered on metal surface.)
Then, metal becomes ion that dissolves into solution, which forms a contaminated substance by reacting to soluble ion in the solution.
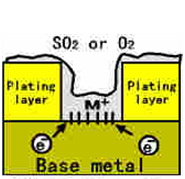 Garubani style Surface Contamination through pin-holemmmmmmmmmmm |
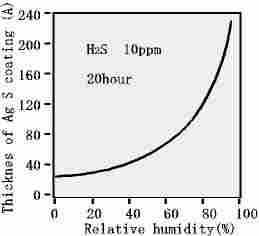 Humidity Influence during corrosion process by H2S of Ag |